Polymyxin Resistance Testing Market: Developments, Growth Drivers, and Predictions for 2025–2035
Overview:
The global polymyxin resistance testing market is poised for consistent expansion, driven by the escalating need to combat antimicrobial resistance. In 2025, the market is anticipated to attain a valuation of USD 137.6 million, propelled by heightened awareness and rigorous diagnostic practices. The projected compound annual growth rate (CAGR) of 6.2% from 2025 to 2035 is expected to elevate the market to USD 250.9 million by the end of the forecast period. This growth trajectory is underpinned by increasing incidences of polymyxin-resistant bacteria across healthcare settings.
Technological advancements in diagnostic methodologies, paralleled by the imperative for rapid and accurate resistance detection, are significantly shaping the market. The adoption of advanced molecular diagnostic tools and automated systems is streamlining testing processes and enhancing the precision of results. These improvements enable healthcare providers to implement timely and targeted therapeutic interventions.
Geographically, North America and Europe are positioned as key markets, owing to well-established healthcare infrastructures and stringent regulatory frameworks. The Asia Pacific region is also anticipated to exhibit substantial growth, driven by increasing healthcare expenditure and the rising prevalence of infectious diseases. Strategic collaborations and partnerships among market participants are further fostering innovation and market penetration.
The competitive landscape is defined by the presence of several prominent players focused on product innovation and strategic expansions. These entities are dedicated to developing cutting-edge solutions that address the evolving demands of the healthcare sector. The future growth of the polymyxin resistance testing market hinges on continuous advancements in diagnostics and a concerted global effort to mitigate antimicrobial resistance.
The market’s progression is also influenced by the imperative for enhanced surveillance and monitoring programs, crucial for tracking the emergence and dissemination of resistant strains. Stakeholders across the healthcare ecosystem are increasingly recognizing the pivotal role of diagnostics in guiding appropriate antimicrobial stewardship practices. These collective endeavors are essential for preserving the efficacy of existing antimicrobial agents and safeguarding public health.
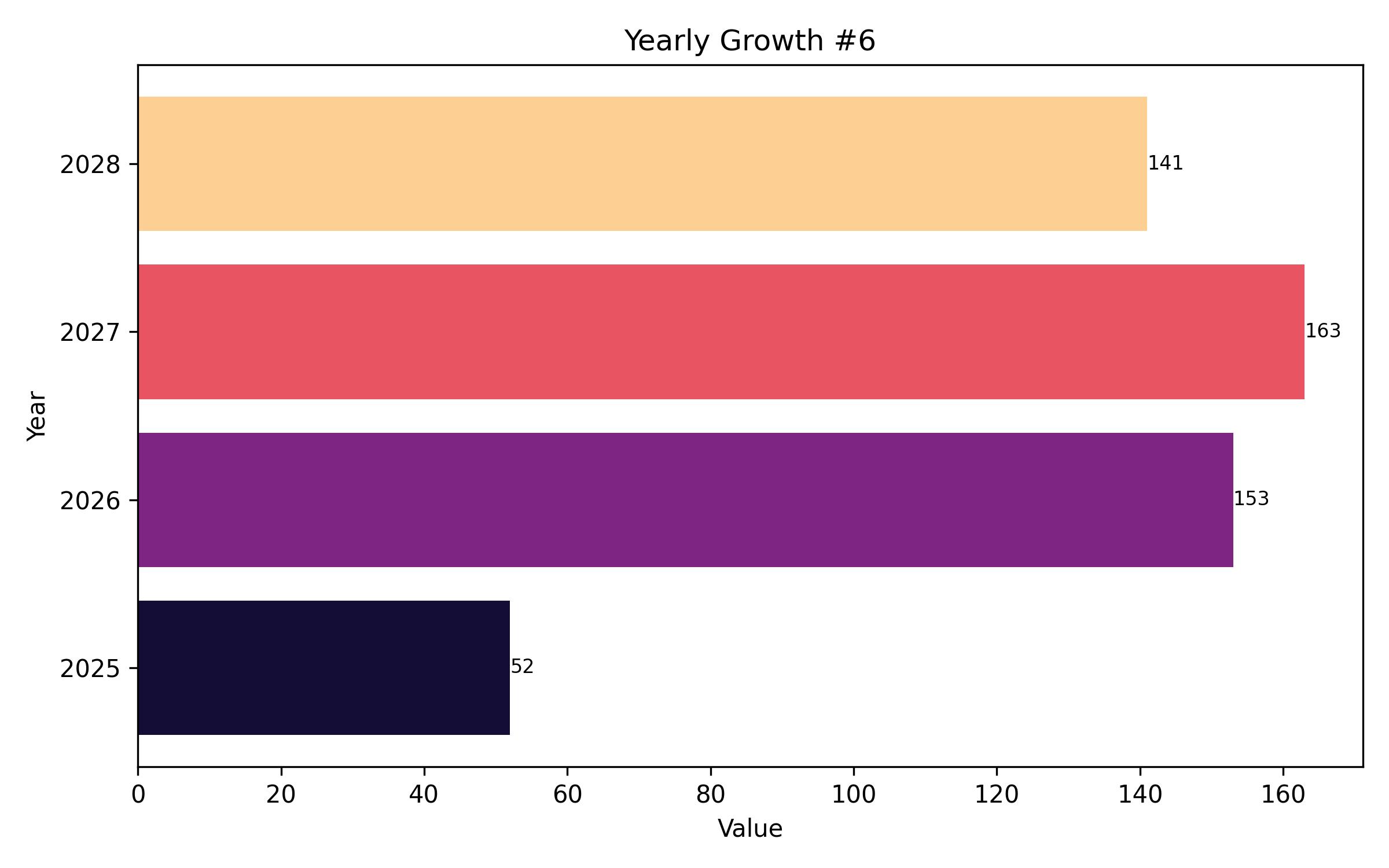
Year On Year Growth Chart
“`html
| Report Attribute | Details |
|---|---|
| Market Size in 2025 | USD 137.6 million |
| Revenue Forecast for 2035 | USD 250.9 million |
| Growth Rate (CAGR) | 6.2% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | Not Available |
| Forecast Period | 2025 – 2035 |
| Quantitative Units | Revenue in USD million and CAGR from 2025 to 2035 |
| Report Coverage | Revenue forecast, market share, competitive landscape, growth factors, and trends |
| Covered Segments | Product, Testing Methods, End User, and Region |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, MEA |
| Country Scope | U.S., Canada, Germany, France, Italy, U.K., Japan, South Korea, New Zealand |
| Key Companies Analyzed | Thermo Fisher Scientific; bioMérieux; BD (Becton, Dickinson and Company); Liofilchem S.r.l.; HiMedia Laboratories; Merck KGaA (MilliporeSigma); ELITechGroup; Creative Diagnostics |
| Customization Options | Free report customization (up to 8 analysts working days) with purchase. Changes to country, regional, and segment scope |
| Pricing and Purchase Options | Customizable purchase options for tailored research needs |
“`
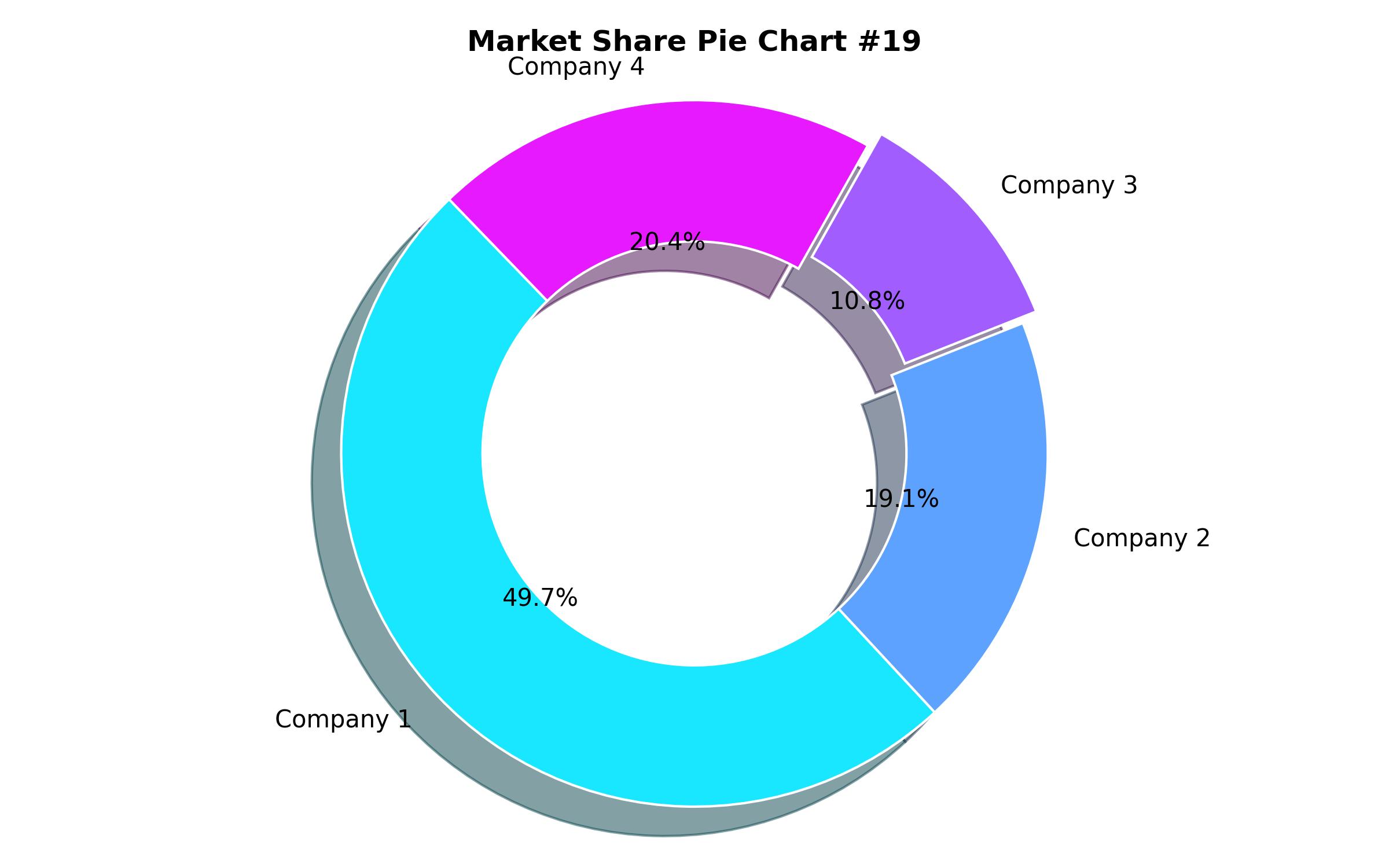
Key Companies Market Share
Report Coverage & Deliverables
- Market Trends And Dynamics
- Competitve Benchmarking
- Historical data and forecasts
- Value/Volume analysis
- Company revenue shares and key strategies
- Regional opportunities
This is an indicative segmentation. Please request a sample report to see detail segmentation of this market.
Detailed Market Segmentation
- By Product
- Consumables
- Instruments
- By Testing Method
- MIC Test
- Disk Diffusion Test
- Automated AST Systems
- Molecular Diagnostic Tests
- By End User
- Hospitals and Diagnostic Centers
- Research and Academic Institutes
- Pharmaceutical and Biotechnology Companies
- Contract Research Organizations
- By Region
- North America (U.S., Canada)
- Europe (Germany, France, U.K., Italy, Spain)
- Asia Pacific (China, Japan, India, South Korea)
- Latin America (Brazil, Mexico)
- Middle East & Africa (Saudi Arabia, UAE)
Table of Content
- Executive Summary
- Market Dynamics
- Key Market Drivers
- Restraints and Challenges
- Market Opportunities
- Product Analysis and Trends
- Testing Methodologies Overview
- End-User Landscape
- Regional Market Analysis
- North America: Market Size and Forecast
- Europe: Market Size and Forecast
- Asia Pacific: Market Size and Forecast
- Latin America: Market Size and Forecast
- Middle East & Africa: Market Size and Forecast
- Competitive Landscape
- Key Company Profiles
- Emerging Technologies in Testing
- Regulatory Framework
- Market Outlook and Projections
- Strategic Recommendations
- Research Methodology
- Appendix
