Nipah Virus (NiV) Infection Testing Market: Analysis by Test Type, End User, and Region Through 2035
Overview:
The global Nipah virus (NiV) infection testing market is poised for steady growth over the coming years. The market is projected to reach USD 2.18 billion in 2025 and is expected to grow to USD 2.63 billion by 2035, reflecting a compound annual growth rate (CAGR) of 5.2% from 2025 to 2035. This growth is primarily driven by the increasing frequency and severity of NiV outbreaks and the subsequent demand for effective diagnostic solutions.
The rise in global travel and trade, coupled with environmental changes, has facilitated the spread of the virus, necessitating robust testing infrastructure. The market is segmented by test type, end user, and region, each contributing uniquely to the overall dynamics.
Molecular tests, particularly RT-PCR, remain the gold standard for accurate and timely detection of NiV infections. Serological tests like ELISA are crucial for epidemiological studies and surveillance. Rapid diagnostic tests are increasingly important for point-of-care applications, enabling quick decision-making in outbreak scenarios.
Hospitals and diagnostic laboratories constitute the primary end-users, while research institutes play a vital role in the development and validation of novel testing methodologies. Point-of-care testing is expected to witness significant growth, driven by the need for decentralized testing capabilities.
Regionally, Asia-Pacific is expected to dominate the market due to the historical prevalence of NiV outbreaks in the region. North America and Europe are also anticipated to show substantial growth, fueled by increased awareness and proactive surveillance programs.
Key companies are focusing on expanding their testing portfolios and enhancing their distribution networks. The market’s future growth will be further propelled by ongoing research, technological advancements, and international collaborations aimed at combating NiV infections.
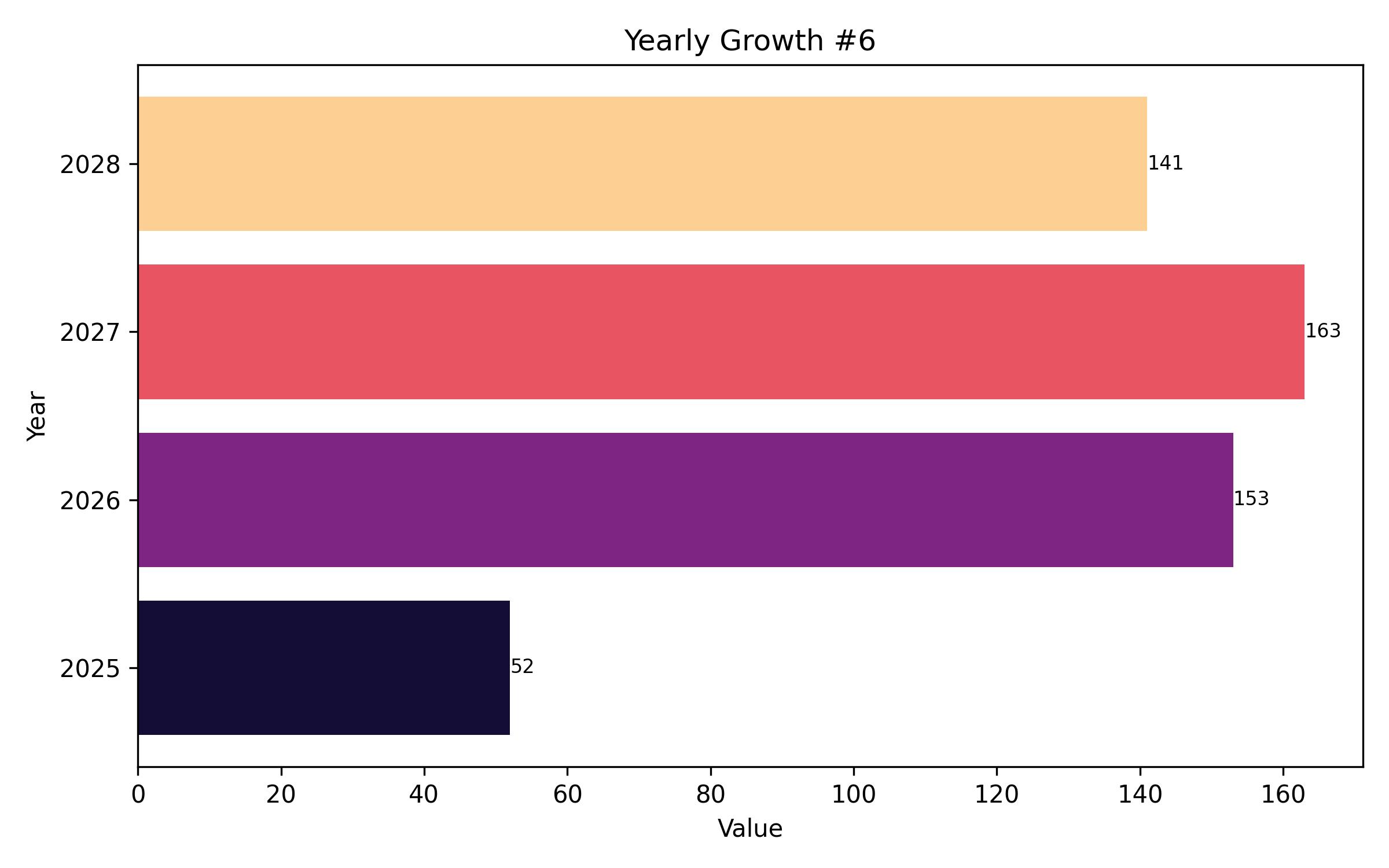
Year On Year Growth Chart
“`html
| Report Attribute | Details |
|---|---|
| Market Size in 2025 | USD 2.18 billion |
| Revenue Forecast for 2035 | USD 2.63 billion |
| Growth Rate (CAGR) | 5.2% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | Not Available |
| Forecast Period | 2025 – 2035 |
| Quantitative Units | Revenue in USD million/billion and CAGR from 2025 to 2035 |
| Report Coverage | Revenue forecast, company market share, competitive landscape, growth factors, and trends |
| Covered Segments | Test Type, End User, and Region |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, MEA |
| Country Scope | U.S., Canada, Mexico, U.K., Germany, Italy, Poland, China, India, Japan, Australia, South Korea, Brazil, UAE, KSA, South Africa |
| Key Companies Analyzed | Thermo Fisher Scientific; Roche Diagnostics; Abbott Laboratories; QIAGEN; Bio-Rad Laboratories |
| Customization Options | Free report customization (up to 8 analysts working days) with purchase. Changes to country, regional, and segment scope |
| Pricing and Purchase Options | Customizable purchase options for tailored research needs |
“`
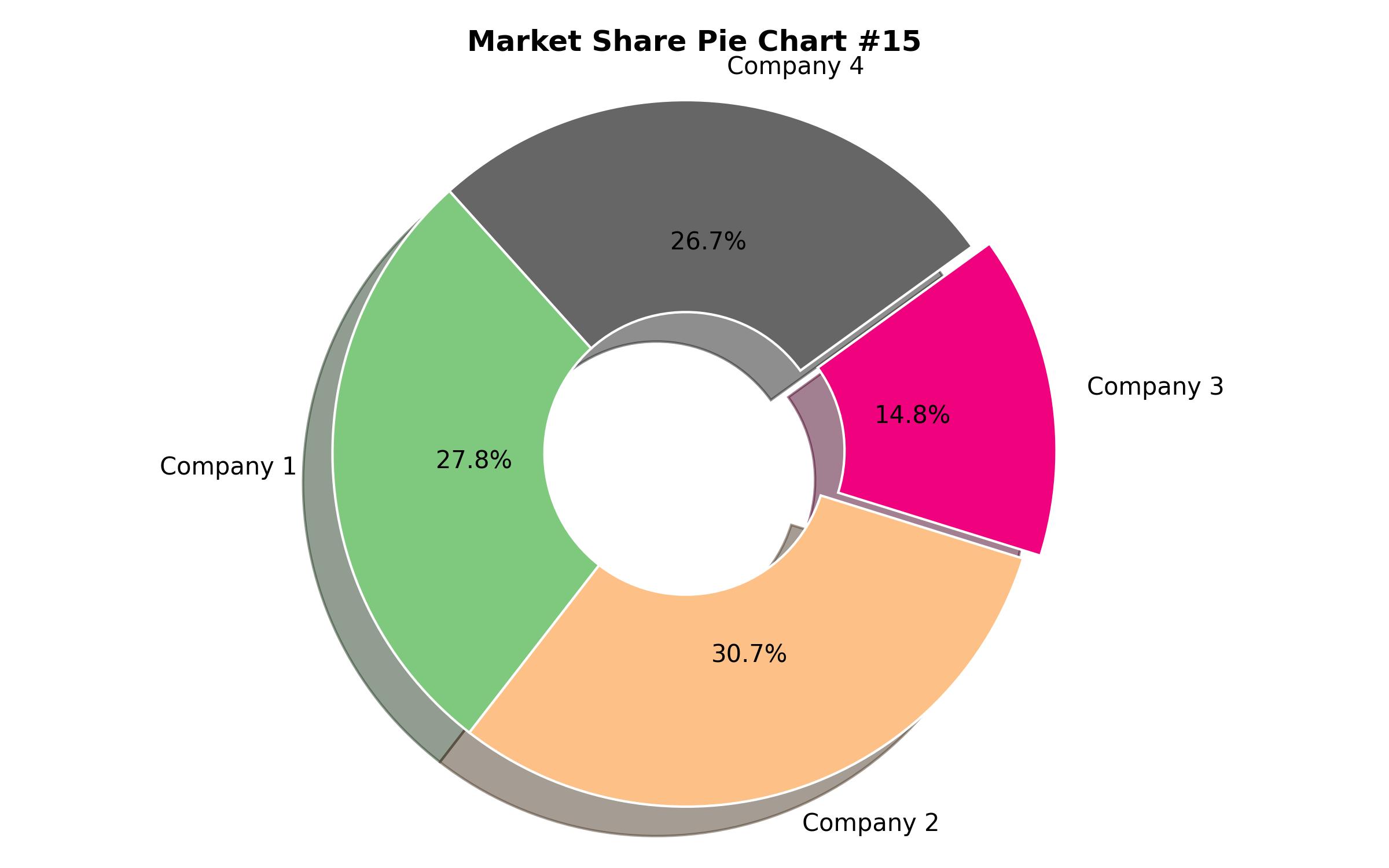
Key Companies Market Share
Report Coverage & Deliverables
- Market Trends And Dynamics
- Competitve Benchmarking
- Historical data and forecasts
- Value/Volume analysis
- Company revenue shares and key strategies
- Regional opportunities
This is an indicative segmentation. Please request a sample report to see detail segmentation of this market.
Detailed Market Segmentation
- By Test Type
- Molecular Tests (RT-PCR)
- Serological Tests (ELISA)
- Rapid Diagnostic Tests
- By End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Research Institutes
- Point of Care Testing
- By Region
- North America (U.S., Canada, Mexico)
- Europe (U.K., Germany, France, Italy, Poland)
- Asia-Pacific (China, India, Japan, Australia, South Korea)
- Latin America (Brazil, Argentina)
- Middle East & Africa (UAE, Saudi Arabia, South Africa)
Table of Content
- Executive Summary
- Market Overview
- Key Market Trends
- Market Dynamics
- Market Drivers
- Market Restraints
- Market Opportunities
- Market Analysis 2025 to 2035, By Test Type
- Molecular Tests (RT-PCR)
- Serological Tests (ELISA)
- Rapid Diagnostic Tests
- Market Analysis 2025 to 2035, By End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Research Institutes
- Point of Care Testing
- Market Analysis 2025 to 2035, By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
- North America Market Analysis 2025 to 2035
- Europe Market Analysis 2025 to 2035
- Asia-Pacific Market Analysis 2025 to 2035
- Latin America Market Analysis 2025 to 2035
- Middle East & Africa Market Analysis 2025 to 2035
- Competitive Landscape
- Company Profiles
- Thermo Fisher Scientific
- Roche Diagnostics
- Abbott Laboratories
- QIAGEN
- Bio-Rad Laboratories
- Market Forecast 2025 to 2035
- Research Methodology
