Comprehensive Analysis of the Prostate-Specific Antigen Testing Market: Assessment of Size, Share, and Future Projections from 2025 to 2035
Overview:
The global prostate-specific antigen (PSA) testing market is poised for substantial expansion in the coming years. In 2025, the market is projected to reach USD 7.9 billion, exhibiting a compound annual growth rate (CAGR) of 12.0%, and is expected to attain a valuation of USD 24.6 billion by 2035. This upswing is attributed to the escalating demand for early and accurate diagnostic tools for prostate-related conditions.
Technological advancements in immunoassay techniques and the development of more sensitive and specific PSA assays are significantly influencing the market. These improvements enable earlier detection and better management of prostate cancer and other prostatic diseases.
Geographically, North America currently leads the market, but the Asia-Pacific region is anticipated to experience the highest growth rate due to increasing healthcare awareness and improving access to diagnostic services. Key industry participants include major diagnostic companies and research institutions.
Market growth is further propelled by increasing awareness campaigns promoting regular check-ups and screenings, coupled with the rising prevalence of prostate cancer globally. These collective factors are expected to sustain the upward trajectory of the global PSA testing market.
The increasing geriatric population, which is more susceptible to prostate disorders, also contributes to market expansion. Additionally, government initiatives supporting cancer screening programs are expected to bolster market growth during the forecast period.
Moreover, continuous research and development efforts aimed at enhancing the accuracy and reliability of PSA tests will likely drive further adoption and market penetration. The focus on personalized medicine and targeted therapies is also expected to create new opportunities in the PSA testing market.
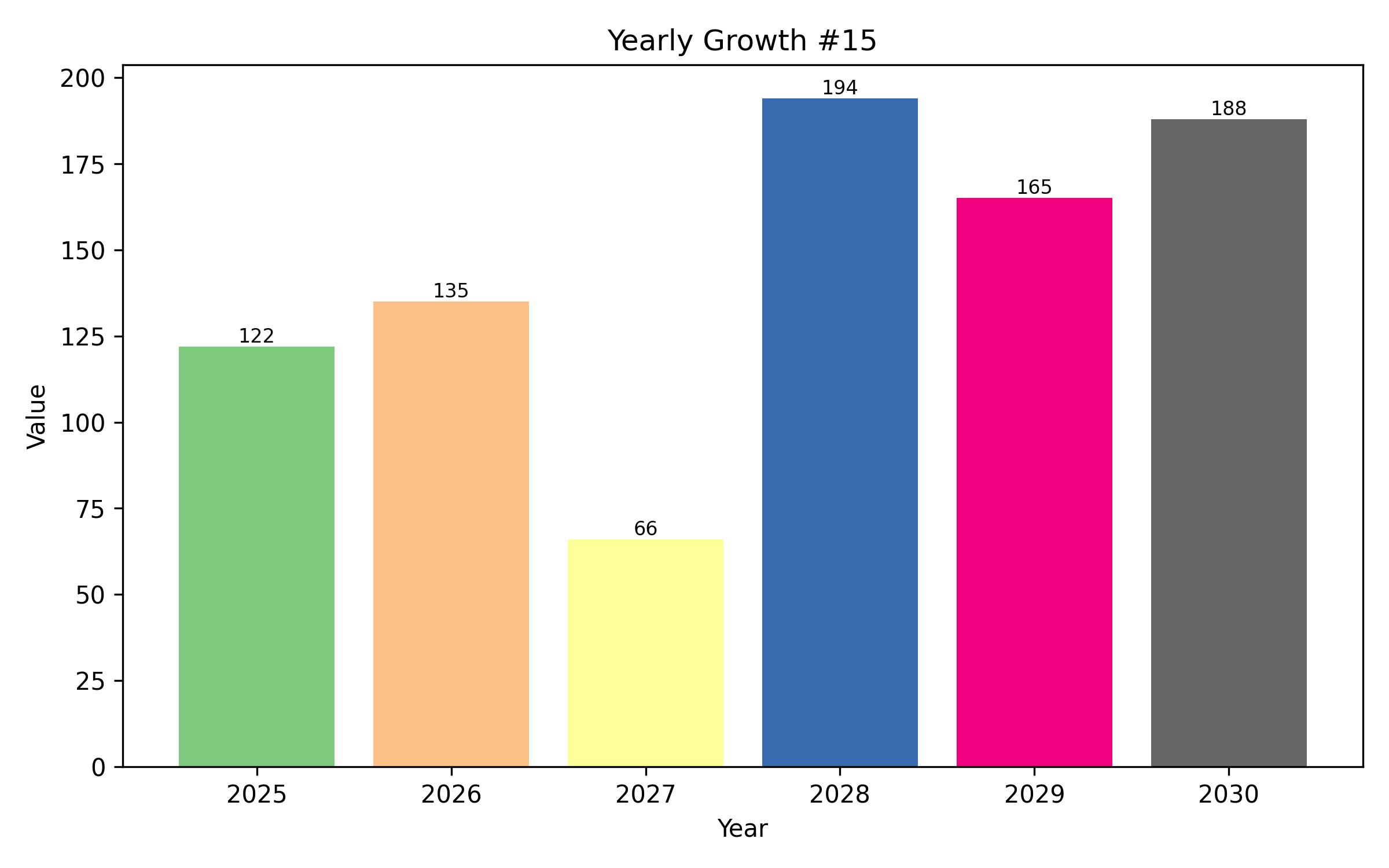
Year On Year Growth Chart
“`html
| Report Attribute | Details |
|---|---|
| Market Size in 2025 | USD 7.9 billion |
| Revenue Forecast for 2035 | USD 24.6 billion |
| Growth Rate (CAGR) | 12.0% from 2025 to 2035 |
| Base Year for Estimation | 2024 |
| Historical Data | 2018 – 2023 |
| Forecast Period | 2025 – 2035 |
| Quantitative Units | Revenue in USD million/billion and CAGR from 2025 to 2035 |
| Report Coverage | Revenue forecast, company market share, competitive landscape, growth factors, and trends |
| Covered Segments | Test Type, Sample Type, End-Use and Region |
| Regional Scope | North America, Europe, Asia Pacific, Latin America, MEA |
| Country Scope | U.S., Canada, Mexico, U.K., Germany, Italy, Poland, China, India, Japan, Australia, South Korea, Brazil, UAE, KSA, South Africa |
| Key Companies Analyzed | Xiamen Biotime Biotechnology Co. Ltd., HWTAi, OptiBio Co., Ltd, Jiangsu MicroDiag Biomedicine Technology Co., Ltd, Beijing Hotgen Biotechn Co., Ltd, Humasis, Accuquik™ Test Kits, CTK Biotech, Inc, INTEC, XIAMEN BOSON BIOTECH CO., LTD, AccuBioTech Co., Ltd, Bio-Rad Laboratories, Inc, Accuquik Test Kits, OPKO Health, Inc, bioMérieux SA, Beckman Coulter, Inc, Abbott, Siemens Healthineers, DiaSorin, F. Hoffmann-La Roche Ltd, Mediwatch (LABORIE), BodiTech, Bristol-Myers Squibb Company, GE Healthcare, Endocare, GlaxoSmithKline, Anixa Biosciences, Ortho Clinical, Fujirebio, Roche Diagnostics, Danaher Corporation, Thermo Fisher Scientific |
| Customization Options | Free report customization (up to 8 analysts working days) with purchase. Changes to country, regional, and segment scope |
| Pricing and Purchase Options | Customizable purchase options for tailored research needs |
“`
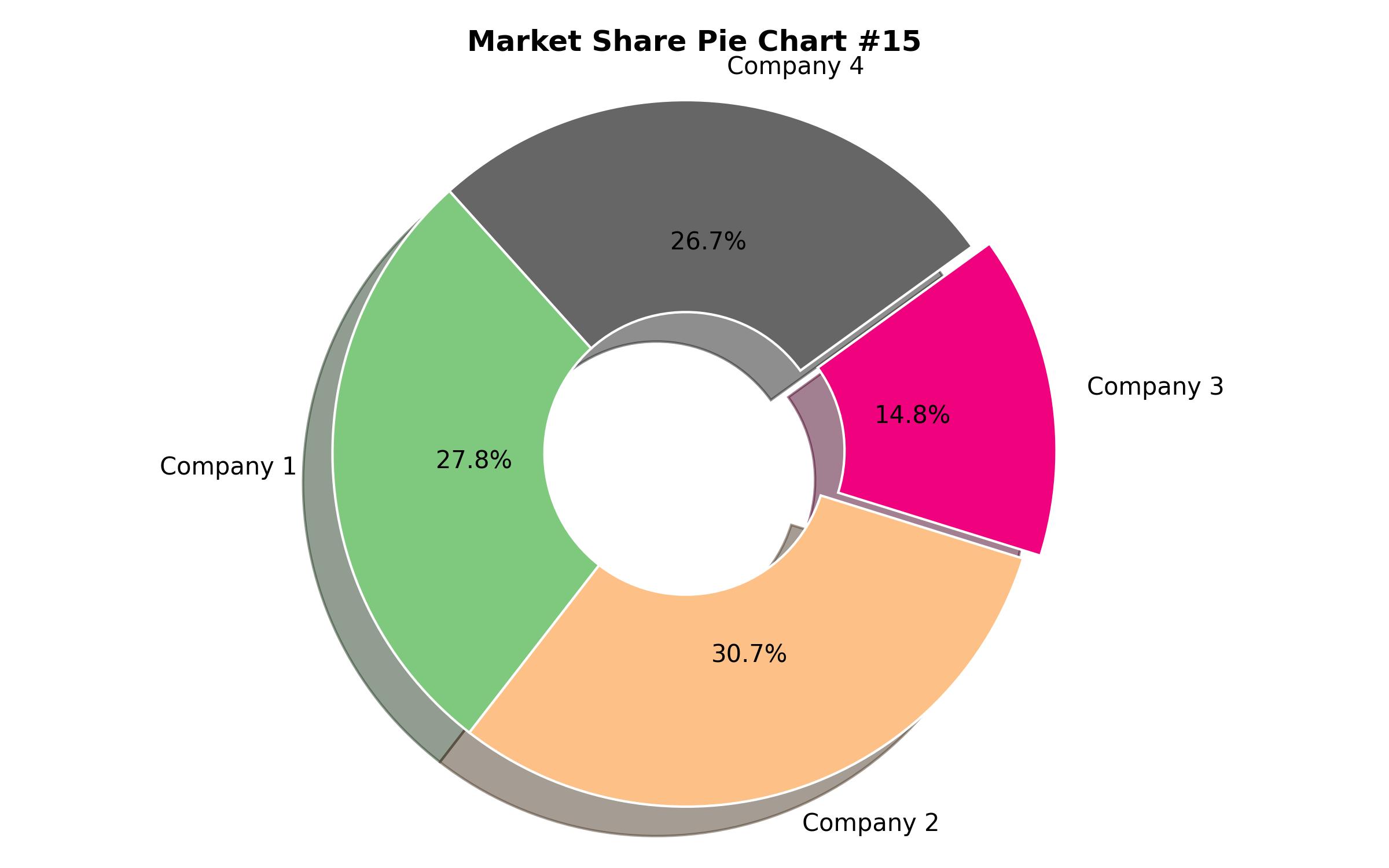
Key Companies Market Share
Report Coverage & Deliverables
- Market Trends And Dynamics
- Competitve Benchmarking
- Historical data and forecasts
- Value/Volume analysis
- Company revenue shares and key strategies
- Regional opportunities
This is an indicative segmentation. Please request a sample report to see detail segmentation of this market.
Detailed Market Segmentation
- By Test Type
- Enzyme Immunoassays (EIAs)
- Radioimmunoassays (RIAs)
- Chemiluminescence Immunoassays (CLIAs)
- Other Test Types
- By Sample Type
- Blood Serum
- Blood Plasma
- Urine
- By End-Use
- Hospitals
- Diagnostic Laboratories
- Research Institutes
- Other End-Users
- By Region
- North America (U.S., Canada, Mexico)
- Europe (U.K., Germany, France, Italy, Poland)
- Asia-Pacific (China, India, Japan, Australia, South Korea)
- Latin America (Brazil, Argentina)
- Middle East & Africa (UAE, Saudi Arabia, South Africa)
Table of Content
- Executive Summary
- Market Overview
- Key Market Trends
- Market Dynamics
- PSA Testing Market Analysis 2025 to 2035, By Test Type
- Enzyme Immunoassays (EIAs)
- Radioimmunoassays (RIAs)
- Chemiluminescence Immunoassays (CLIAs)
- Other Test Types
- PSA Testing Market Analysis 2025 to 2035, By Sample Type
- Blood Serum
- Blood Plasma
- Urine
- PSA Testing Market Analysis 2025 to 2035, By End-Use
- Hospitals
- Diagnostic Laboratories
- Research Institutes
- Other End-Users
- PSA Testing Market Analysis 2025 to 2035, By Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
- North America PSA Testing Market Analysis 2025 to 2035
- Europe PSA Testing Market Analysis 2025 to 2035
- Asia-Pacific PSA Testing Market Analysis 2025 to 2035
- Latin America PSA Testing Market Analysis 2025 to 2035
- Middle East & Africa PSA Testing Market Analysis 2025 to 2035
- Competitive Landscape
- Key Company Profiles
- Market Drivers
- Market Restraints
- Market Opportunities
- Future Trends
- Regulatory Framework
- Analyst Views
- Research Methodology
